Resubmitting Recertifications
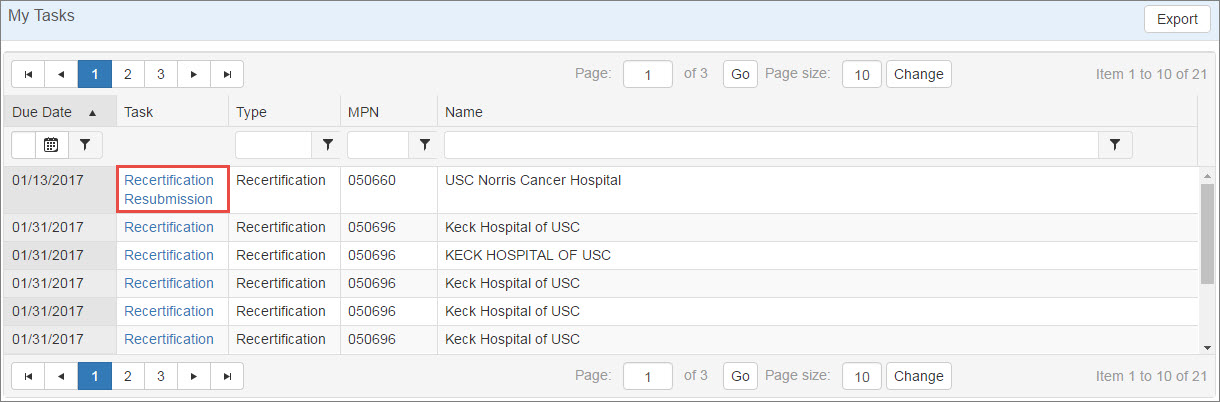
If a recertification has issues that need to be resolved, OPA may elect to return it for correction. You will receive an "AO Task Notification" email that you have a pending task awaiting attention, and the task will create a  Recertification 340B covered entities must annually recertify their eligibility to remain in the 340B Program and continue purchasing covered outpatient drugs at discounted 340B prices. As part of this process, the Authorizing Official of each 340B covered entity certifies basic information about the entity and its 340B compliance. Covered entities with inaccurate information in the 340B OPAIS run a high risk of being removed from the program. Resubmission task in the My Tasks tab of My Dashboard.
Recertification 340B covered entities must annually recertify their eligibility to remain in the 340B Program and continue purchasing covered outpatient drugs at discounted 340B prices. As part of this process, the Authorizing Official of each 340B covered entity certifies basic information about the entity and its 340B compliance. Covered entities with inaccurate information in the 340B OPAIS run a high risk of being removed from the program. Resubmission task in the My Tasks tab of My Dashboard.
This table can be sorted and filtered to make the data displayed more manageable. For more information, refer to Data Tables.
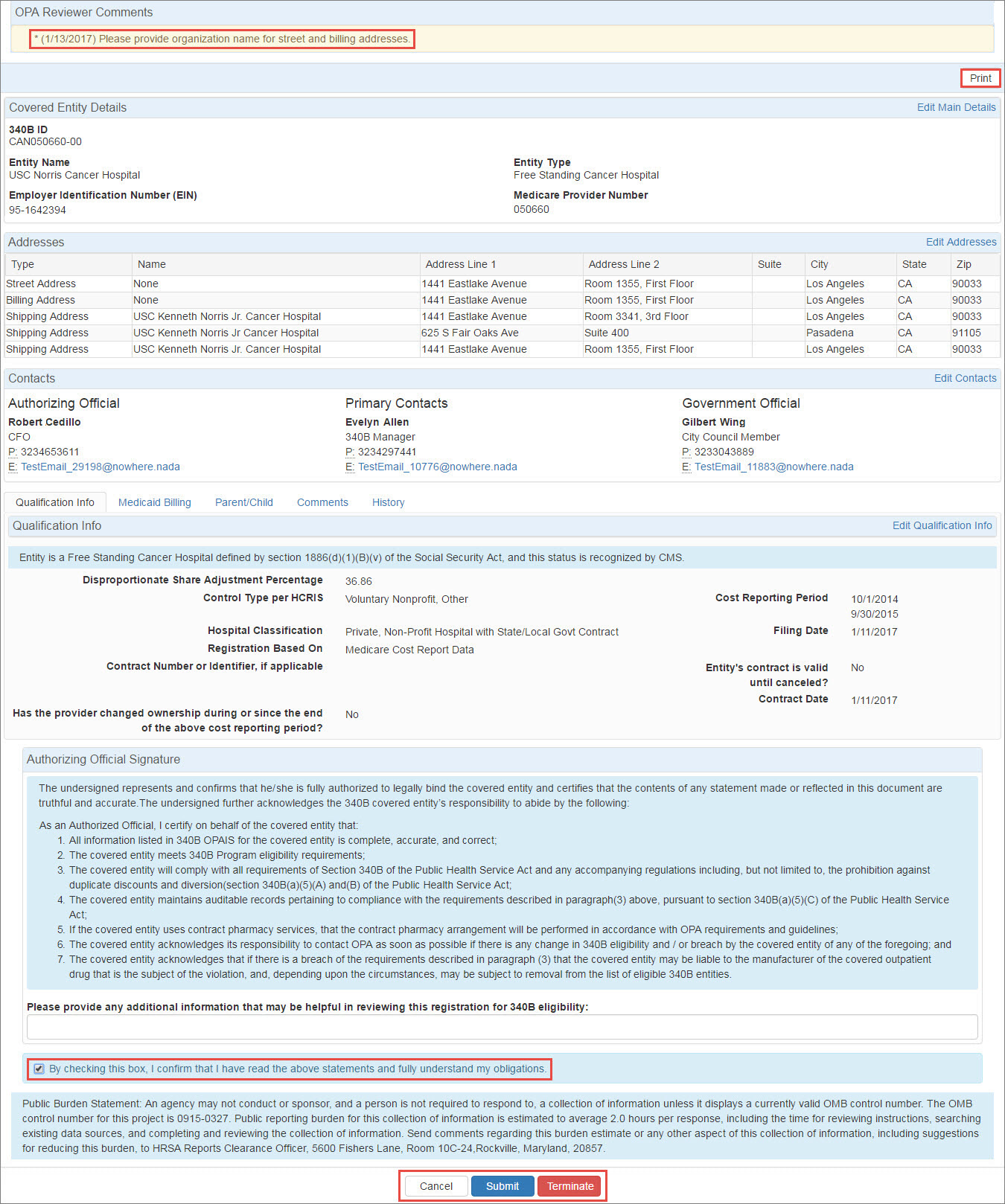
Upon opening the recertification, the OPA Reviewer Comments area will provide instructions for resolving the issue. Click the Edit… link for the appropriate section to edit its information.
| Control | Description |
|---|---|
|
Textbox |
"Please provide any additional information that may be helpful in reviewing this registration for 340B eligibility:" Type any comments that will help OPA in approving the recertification. |
|
Checkbox |
"By checking this box, I confirm that I have read the above statements and fully understand my obligations." Select this box if you intend to submit the recertification to OPA for approval. |
|
Cancel |
Exit from the attestation page without taking action. |
|
Submit |
Click this button to submit the recertification for OPA approval.
|
|
Terminate |
Click this button to terminate the entity. The Termination Information page will be displayed for entry of the termination reason, dates, and comments. |
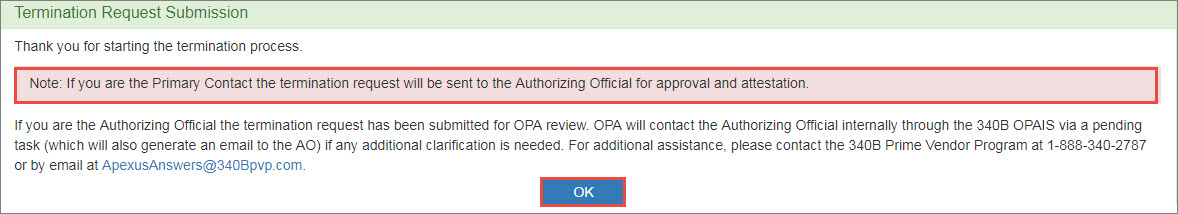
Upon clicking the Submit button, the Recertification Submission confirmation page is displayed.
Click the OK button to return to the My Tasks tab of My Dashboard.