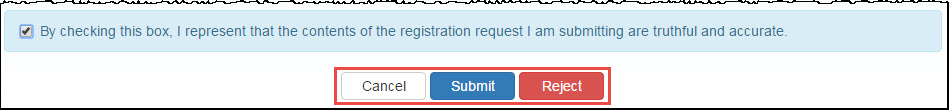
Attesting to Labeler Code Registrations
When you elect to attest to a registration submitted by a Primary Contact, the attestation statement is appended to the bottom of the summary page. You must attest to the registration before it can be submitted to OPA for approval.
| Control | Description |
|---|---|
|
Checkbox |
"By checking this box, I represent that the contents of the registration request I am submitting are truthful and accurate." Select this box if you intend to submit the registration to OPA for approval. |
|
Cancel |
Exit from the attestation page without taking action. |
|
Submit |
Click this button to submit the registration for OPA approval. |
|
Reject |
This button is displayed if the registration was submitted by a Primary Contact. Clicking this button will reject the registration and remove it from the My Tasks tab of My Dashboard. |
-
Click the Continue button. The system will display a PDF version of the Pharmaceutical Pricing Agreement (
 PPA This agreement is required for manufacturers who have executed a Medicaid rebate agreement with CMS. It is voluntary for those who do not have a current Medicaid rebate agreement. The PPA must be signed by a corporate officer of the company (e.g., president, chief executive officer, or general counsel). Signatures by vice presidents or directors of sales or marketing will not be accepted. A PPA remains in effect until terminated by either the manufacturer or the Secretary of HHS. It is not automatically terminated if a manufacturer terminates its Medicaid rebate agreement.) with a "Draft" watermark. You will not be able to view a countersigned version of the PPA until after review and acceptance by OPA.
PPA This agreement is required for manufacturers who have executed a Medicaid rebate agreement with CMS. It is voluntary for those who do not have a current Medicaid rebate agreement. The PPA must be signed by a corporate officer of the company (e.g., president, chief executive officer, or general counsel). Signatures by vice presidents or directors of sales or marketing will not be accepted. A PPA remains in effect until terminated by either the manufacturer or the Secretary of HHS. It is not automatically terminated if a manufacturer terminates its Medicaid rebate agreement.) with a "Draft" watermark. You will not be able to view a countersigned version of the PPA until after review and acceptance by OPA.If you are using Google Chrome as your web browser, you will need to install a PDF viewer to view the PPA and addendum.
-
Read the instructions for completing the PPA and review the information in the IX. Signatures section at the bottom of the document. Your name and contact information will appear as the signatory in the Accepted for the Manufacturer section.
PPA Controls Control Description Checkbox
"I acknowledge reviewing the 340B Drug Pricing Program Pharmaceutical Pricing Agreement (PPA) and attest the information submitted is truthful and accurate."
Select this box if you intend to submit the registration to OPA for approval.
Edit
Return to the Manufacturer Labeler Code(s) page if you need to make changes.
Cancel
Cancel the registration and return to your My Tasks page.
Continue
Accept the PPA and view the PPA Addendum.
-
Click the Continue button. The system will display a PDF version of the PPA Addendum with a "Draft" watermark. You will not be able to view a countersigned version of the PPA Addedum until after review and acceptance by OPA.
-
Read the instructions for completing the PPA and review the information in the IX. Signatures section at the bottom of the document. Your name and contact information will appear as the signatory in the Accepted for the Manufacturer section.
PPA Addendum Controls Controls Description Checkbox
"I acknowledge reviewing the 340B Drug Pricing Program Pharmaceutical Pricing Agreement (PPA) Addendum and attest the information submitted is truthful and accurate."
Select this box if you intend to submit the registration to OPA for approval.
Back
Return to the Pharmaceutical Pricing Agreement (PPA) page.
Cancel
Cancel the registration and return to your My Tasks page.
Continue
Accept the PPA Addendum.
-
Click the Continue button. The system will display the Submit Manufacturer Registration page to confirm the electronic signatures of the PPA and Addendum.
Manufacturer Registration Submission Controls Control Description Checkbox
"By checking this box, I, <User First and Last Name>, agree to electronically sign this agreement as a part of the online MFR labeler registration and attest the information submitted is truthful and accurate."
Select this box if you intend to submit the registration to OPA for approval.
Back
Return to the Pharmaceutical Pricing Agreement (PPA) page.
Cancel
Cancel the registration and return to your My Tasks page.
Continue
Electronically sign and submit the PPA and Addendum.
-
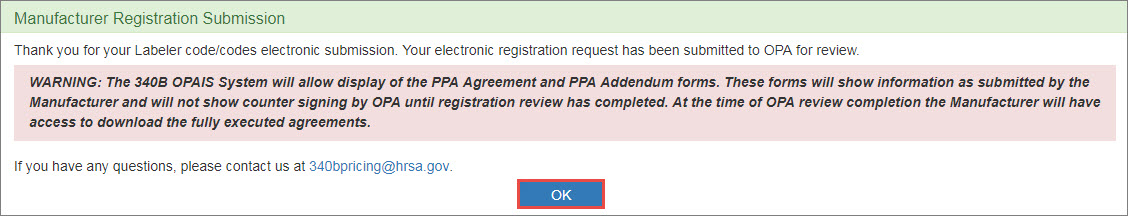
The system will display the Manufacturer Registration Submission confirmation message. Click the OK button to return to the 340B home page.
Upon OPA approval, the AO and the PC will both receive a Manufacturer 340B Participation Approval email and the labeler code's manufacturer status will be updated to "Approved."
-
Click the Continue button. The system will display a PDF version of the Pharmaceutical Pricing Agreement (PPA) with a "Draft" watermark. You will not be able to view a countersigned version of the PPA until after review and acceptance by OPA.
If you are using Google Chrome as your web browser, you will need to install a PDF viewer to view the PPA and addendum.
-
Read the instructions for completing the PPA and review the information in the IX. Signatures section at the bottom of the document. Your name and contact information will appear as the signatory in the Accepted for the Manufacturer section.
PPA Controls Control Description Checkbox
"I acknowledge reviewing the 340B Drug Pricing Program Pharmaceutical Pricing Agreement (PPA) and attest the information submitted is truthful and accurate."
Select this box if you intend to submit the registration to OPA for approval.
Edit
Return to the Manufacturer Labeler Code(s) page if you need to make changes.
Cancel
Cancel the registration and return to your My Tasks page.
Continue
Accept the PPA and view the PPA Addendum.
-
Click the Continue button. The system will display a PDF version of the PPA Addendum with a "Draft" watermark. You will not be able to view a countersigned version of the PPA Addedum until after review and acceptance by OPA.
-
Read the instructions for completing the PPA and review the information in the IX. Signatures section at the bottom of the document. Your name and contact information will appear as the signatory in the Accepted for the Manufacturer section.
PPA Addendum Controls Controls Description Checkbox
"I acknowledge reviewing the 340B Drug Pricing Program Pharmaceutical Pricing Agreement (PPA) Addendum and attest the information submitted is truthful and accurate."
Select this box if you intend to submit the registration to OPA for approval.
Back
Return to the Pharmaceutical Pricing Agreement (PPA) page.
Cancel
Cancel the registration and return to your My Tasks page.
Continue
Accept the PPA Addendum.
-
Click the Continue button. The system will display the Submit Manufacturer Registration page to confirm the electronic signatures of the PPA and Addendum.
Manufacturer Registration Submission Controls Control Description Checkbox
"By checking this box, I, <User First and Last Name>, agree to electronically sign this agreement as a part of the online MFR labeler registration and attest the information submitted is truthful and accurate."
Select this box if you intend to submit the registration to OPA for approval.
Back
Return to the Pharmaceutical Pricing Agreement (PPA) page.
Cancel
Cancel the registration and return to your My Tasks page.
Continue
Electronically sign and submit the PPA and Addendum.
-
The system will display the Manufacturer Registration Submission confirmation message. Click the OK button to return to the 340B home page.
Upon OPA approval, the AO and the PC will both receive a Manufacturer 340B Participation Approval email and the labeler code's manufacturer status will be updated to "Approved."