Requesting Changes to a Labeler Code
This function lets you change the manufacturer information and contact information associated with an active labeler code for which you are the Authorizing Official or Primary contact.
-
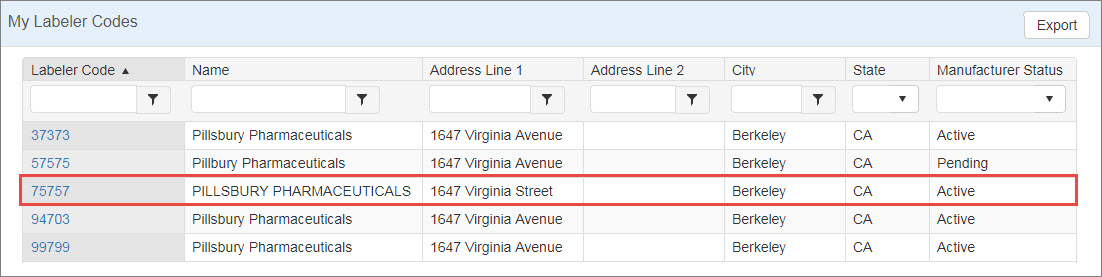
From the My Labeler Codes tab of My Dashboard, click the labeler code you want to change.
-
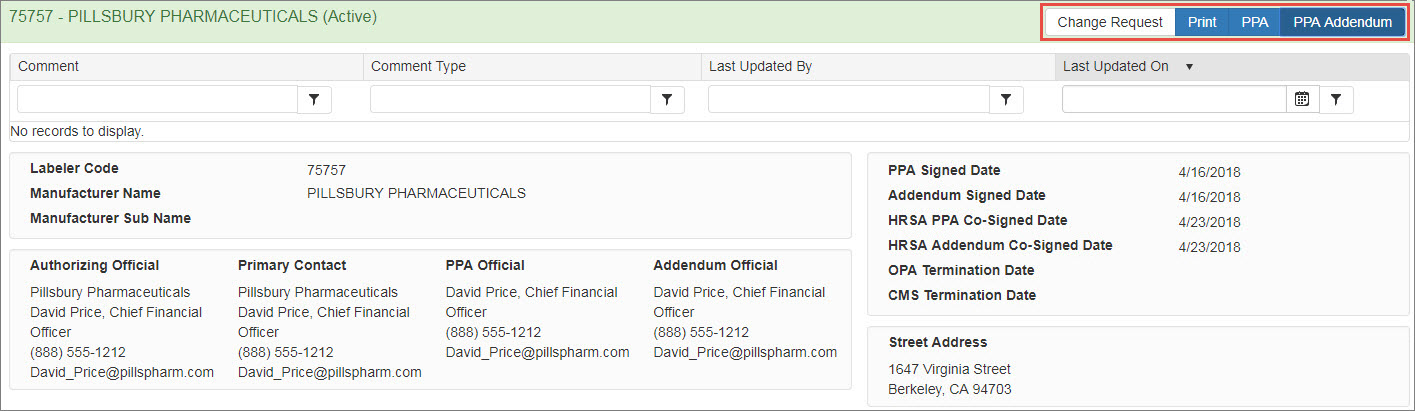
The system displays the manufacturer details page for the labeler code. Click the Change Request button.
The Change Request button is not displayed if the labeler code has a pending change request.
-
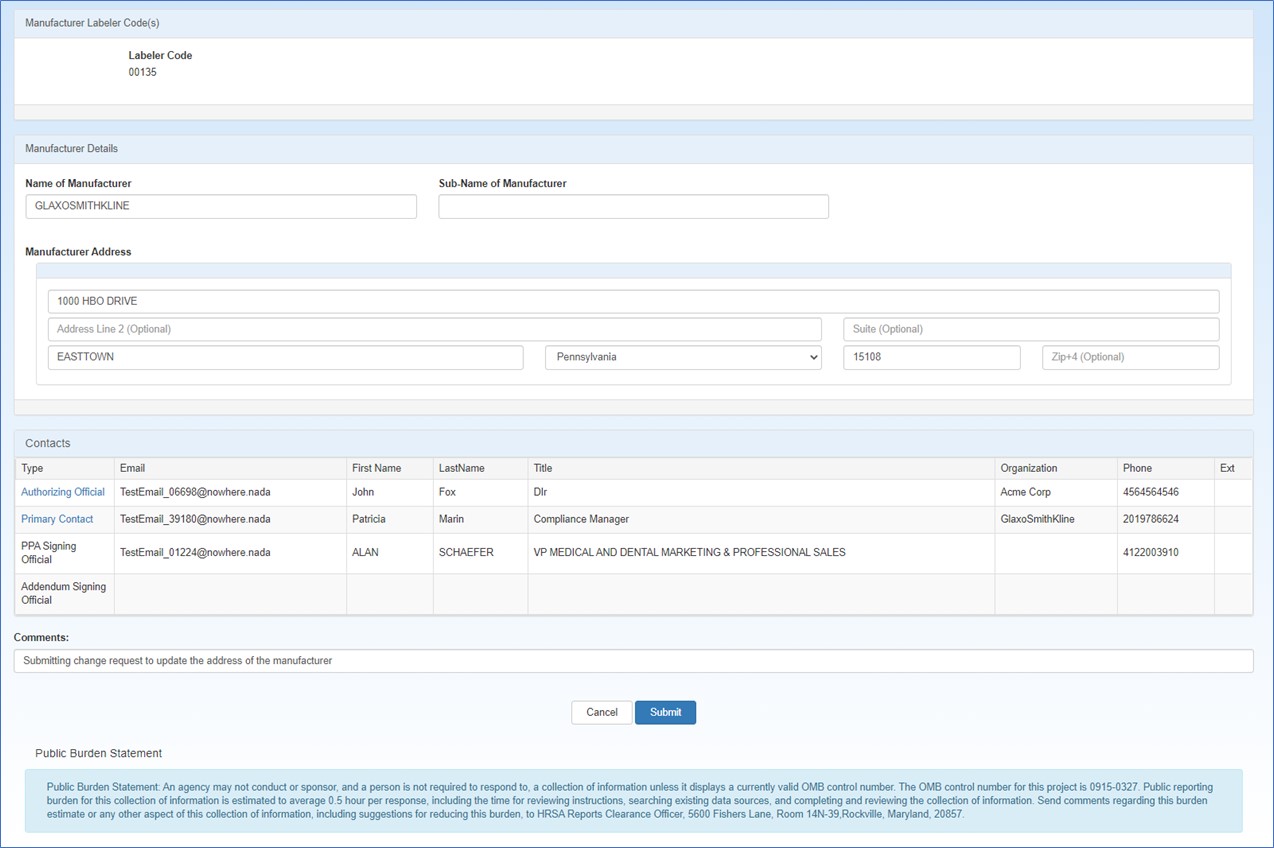
The Labeler Code(s) Change Request page will display the entire labeler code registration.
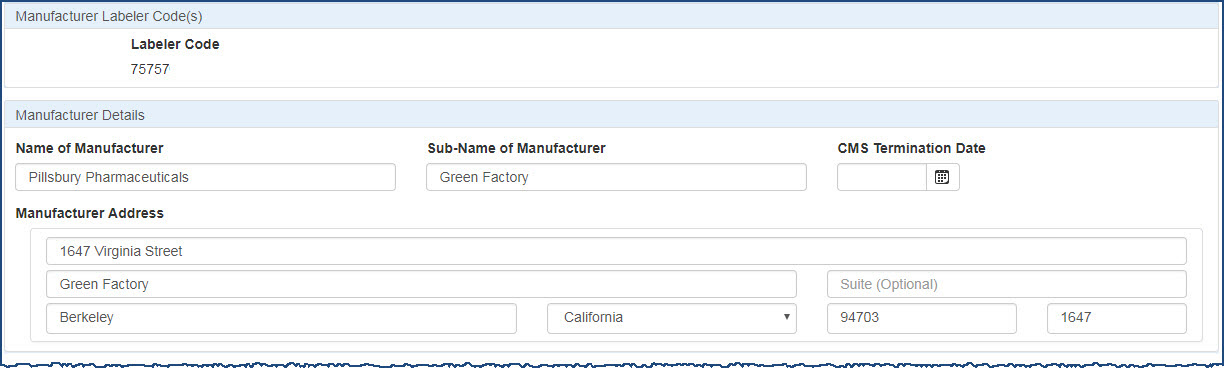
Use the Manufacturer Details section to update the manufacturer's name and address information as necessary.
- The Labeler Code may not be changed.
- The Name of Manufacturer and Sub-Name of Manufacturer (subsidiary) may be updated as necessary.
- Enter the
 CMS Centers for Medicare and Medicaid Services, the federal agency within Health and Human Services (HHS) that administers the Medicare and Medicaid programs, including the Medicaid drug rebate program and the Medicare Part D prescription drug benefit.
CMS Centers for Medicare and Medicaid Services, the federal agency within Health and Human Services (HHS) that administers the Medicare and Medicaid programs, including the Medicaid drug rebate program and the Medicare Part D prescription drug benefit.  Termination Date The date in the 340B OPAIS on which a provider's participation in the 340B program is terminated. After its termination date, a provider can no longer purchase 340B drugs. OPA updates termination dates on a quarterly basis. if the labeler code has been terminated from CMS.
Termination Date The date in the 340B OPAIS on which a provider's participation in the 340B program is terminated. After its termination date, a provider can no longer purchase 340B drugs. OPA updates termination dates on a quarterly basis. if the labeler code has been terminated from CMS. - All of the Manufacturer Address fields may be changed.
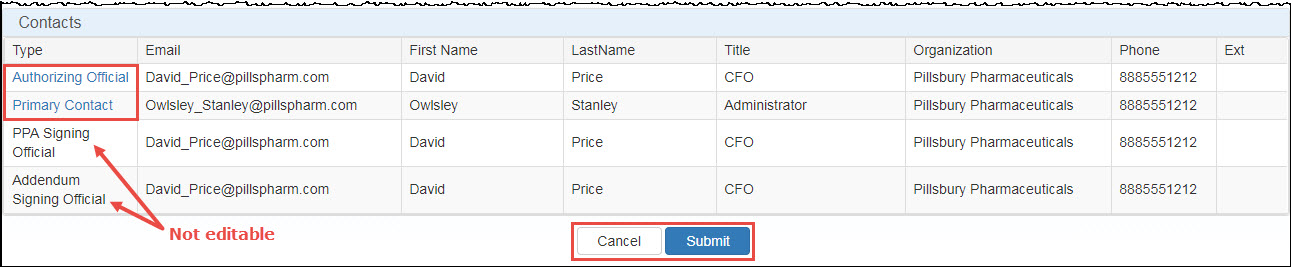
Use the Contacts section to update the contact information for the manufacturer's AO or ![]() PC External user who is designated as a Primary Contact for an entity. This user can enter registrations and update entity information. This user can enter registrations, and update entity information. Any changes to an entity performed by the PC user must be attested to by the AO for that entity.. The
PC External user who is designated as a Primary Contact for an entity. This user can enter registrations and update entity information. This user can enter registrations, and update entity information. Any changes to an entity performed by the PC user must be attested to by the AO for that entity.. The  PPA This agreement is required for manufacturers who have executed a Medicaid rebate agreement with CMS. It is voluntary for those who do not have a current Medicaid rebate agreement. The PPA must be signed by a corporate officer of the company (e.g., president, chief executive officer, or general counsel). Signatures by vice presidents or directors of sales or marketing will not be accepted. A PPA remains in effect until terminated by either the manufacturer or the Secretary of HHS. It is not automatically terminated if a manufacturer terminates its Medicaid rebate agreement. Signing Official and Addendum Signing Official information may not be changed.
PPA This agreement is required for manufacturers who have executed a Medicaid rebate agreement with CMS. It is voluntary for those who do not have a current Medicaid rebate agreement. The PPA must be signed by a corporate officer of the company (e.g., president, chief executive officer, or general counsel). Signatures by vice presidents or directors of sales or marketing will not be accepted. A PPA remains in effect until terminated by either the manufacturer or the Secretary of HHS. It is not automatically terminated if a manufacturer terminates its Medicaid rebate agreement. Signing Official and Addendum Signing Official information may not be changed.
PPA Signing Official and Addendum Signing Offiical are not editable. They are static fields that show a point in time when the oriingal signatories signed the PPA and Addendum
-
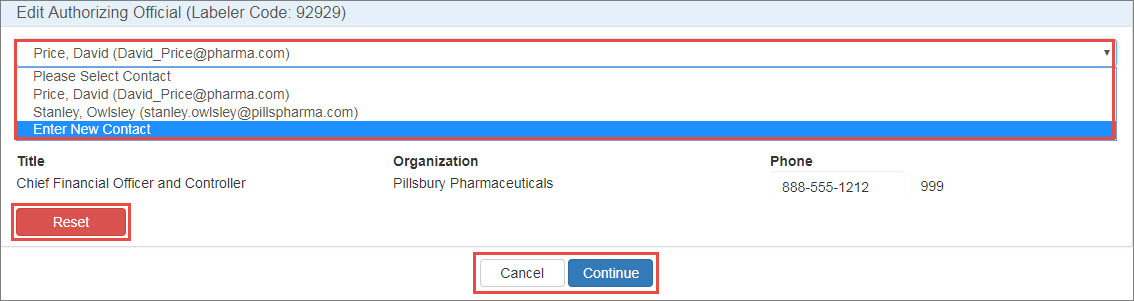
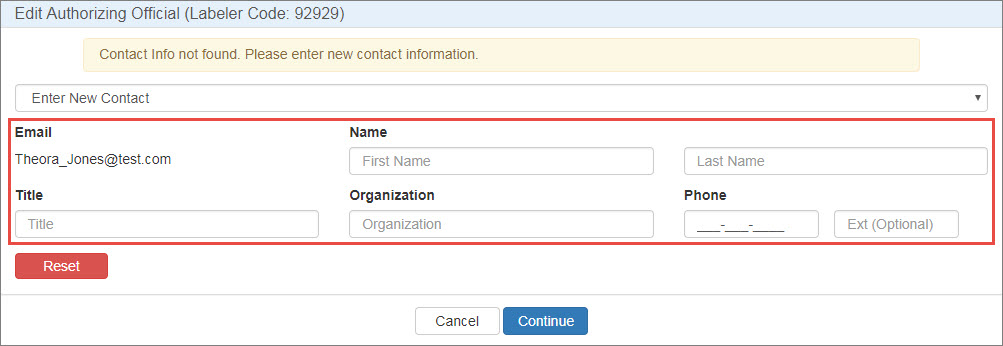
In the Type column, click either the Authorizing Official or Primary Contact link. To change the AO or PC, click the Please Select Contact drop-down list to select the existing AO or PC or to enter a new contact.
-
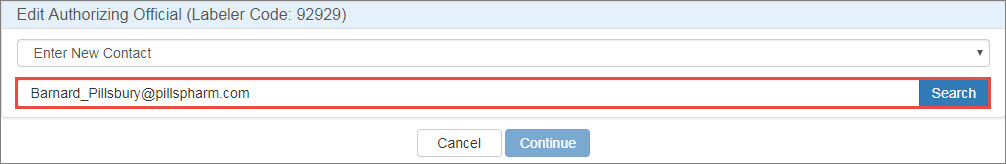
Select Enter New Contact, type the contact's email address, and click the Search button.
-
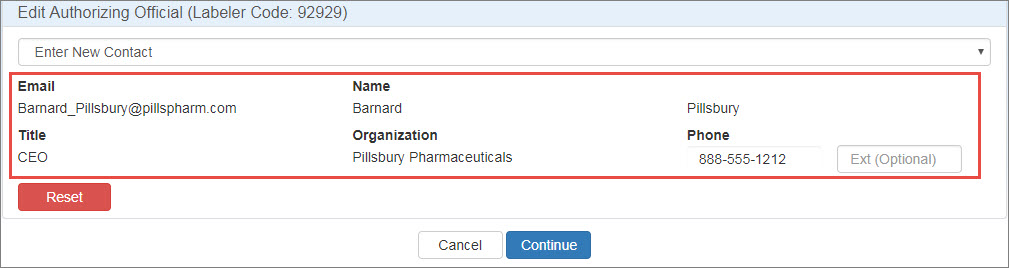
If the contact has a 340B account, their name, title, organization, and phone number will be filled automatically.
-
If the contact does not have a 340B account, type their name, title, organization, and phone number in the spaces provided. They will receive a New User Account Invitation email asking them to create an account.
AO Contact Information Controls Control Description Reset
Clear any existing AO or PC contact information.
Cancel
Cancel the changes and return to the home page.
-
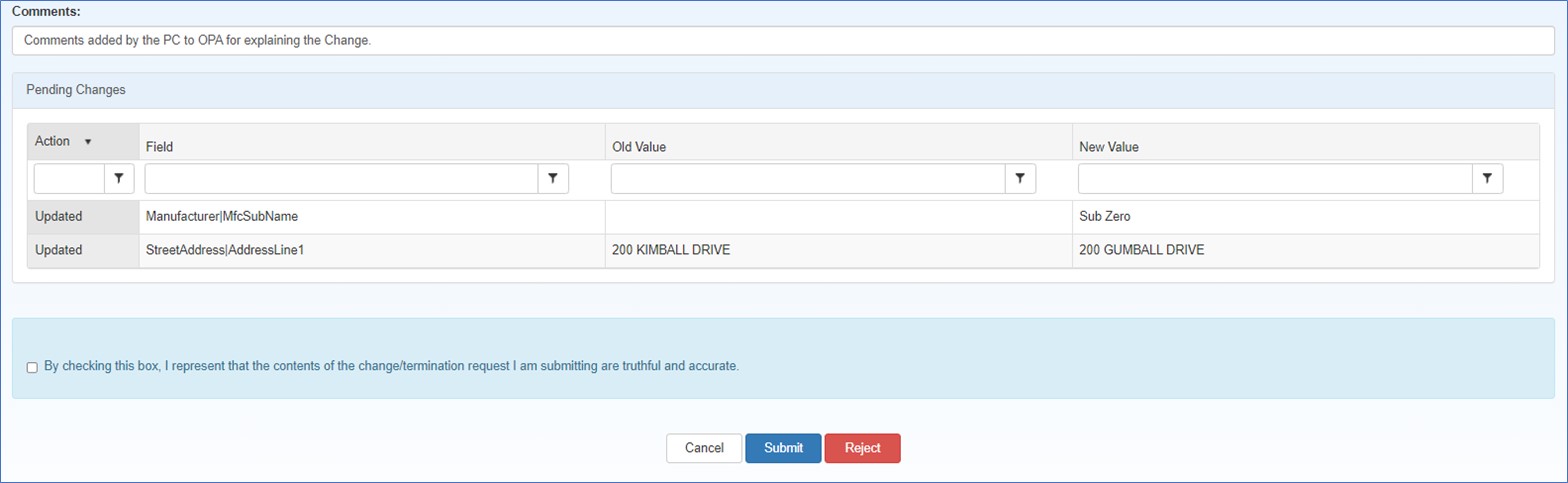
Type any comments that will help OPA in approving the submission. This comment will be displayed in the attestation task submitted to the AO for approval.
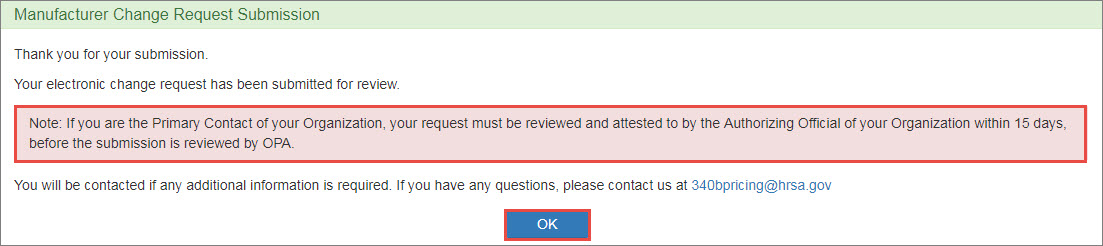
Upon clicking Submit button, the system will display the Manufacturer Change Request Submission confirmation message. Click the OK button to return to your My Dashboard.
The AO must attest to the change request before it can be submitted for OPA approval.
- Upon OPA approval, the system will send an Approval of online 340B Manufacturer Change Request email to the AO and PC.
- If OPA rejects the change request, the system will send a Rejection of online 340B Manufacturer Change Request email to the AO and PC.
The attestation section is appended to the bottom of the summary page. Comments added by the PC regarding the submission will be displayed in the Comments text box. The AO can review and edit the comments.
| Control | Description |
|---|---|
|
Checkbox |
"By checking this box, I represent that the contents of the change/termination request I am submitting are truthful and accurate." Select this box if you intend to submit the change request to OPA for approval. |
|
Cancel |
Exit from the attestation page without taking action. |
|
Submit |
After selecting the checkbox to authorize the submission, click this button to submit the change request for OPA approval. The Manufacturer Registration Submission confirmation page will be displayed.
|
Upon clicking Submit button, the system will display the Manufacturer Change Request Submission confirmation message. Click the OK button to return to your My Dashboard.
The AO must attest to the change request before it can be submitted for OPA approval.
- Upon OPA approval, the system will send an Approval of online 340B Manufacturer Change Request email to the AO and PC.
- If OPA rejects the change request, the system will send a Rejection of online 340B Manufacturer Change Request email to the AO and PC.