My Pending Labeler Codes
The My Pending tab of My Dashboard lists the Labeler Code registrations that have been submitted to OPA, but are awaiting OPA's approval.
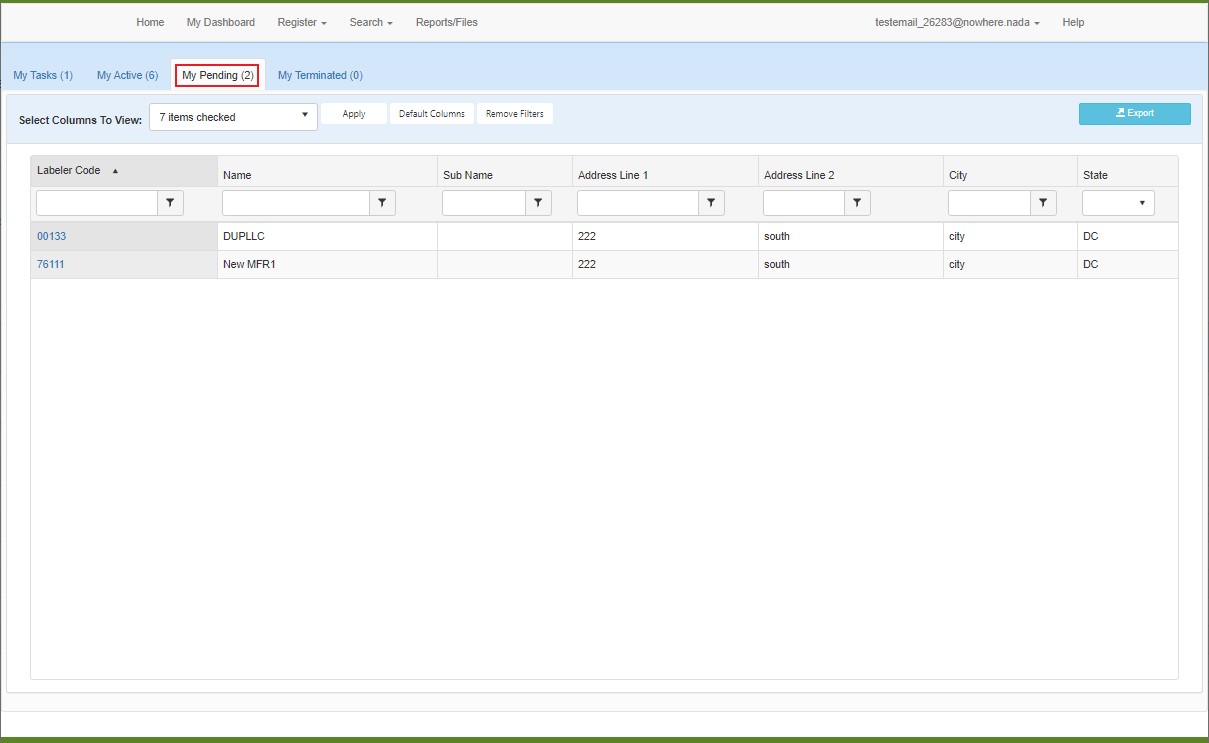
This table can be sorted and filtered to make the data displayed more manageable. For more information, refer to Data Tables.
Click on the hyperlink in the Labeler Code column to open the associated labeler code detail page.
The status of the Labeler Code is reflected by the color of the page header:
- Active (green)
- Pending (blue)
- Terminated (red) – terminated in 340B with an OPA
 Termination Date The date in the 340B OPAIS on which a provider's participation in the 340B program is terminated. After its termination date, a provider can no longer purchase 340B drugs. OPA updates termination dates on a quarterly basis.
Termination Date The date in the 340B OPAIS on which a provider's participation in the 340B program is terminated. After its termination date, a provider can no longer purchase 340B drugs. OPA updates termination dates on a quarterly basis.
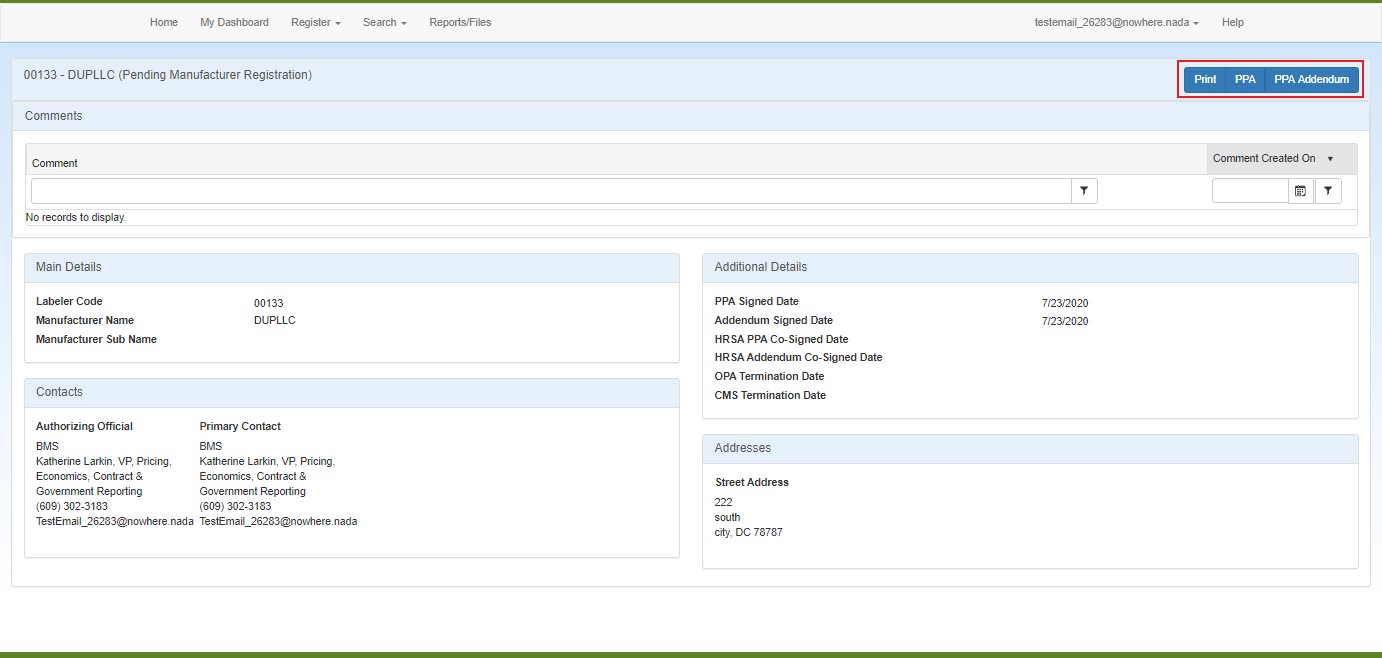
Click the Print button to generate a printer-friendly copy of the page in a separate browser tab.
Click the  PPA This agreement is required for manufacturers who have executed a Medicaid rebate agreement with CMS. It is voluntary for those who do not have a current Medicaid rebate agreement. The PPA must be signed by a corporate officer of the company (e.g., president, chief executive officer, or general counsel). Signatures by vice presidents or directors of sales or marketing will not be accepted. A PPA remains in effect until terminated by either the manufacturer or the Secretary of HHS. It is not automatically terminated if a manufacturer terminates its Medicaid rebate agreement. or PPA Addendum buttons to view a PDF version of either document.
PPA This agreement is required for manufacturers who have executed a Medicaid rebate agreement with CMS. It is voluntary for those who do not have a current Medicaid rebate agreement. The PPA must be signed by a corporate officer of the company (e.g., president, chief executive officer, or general counsel). Signatures by vice presidents or directors of sales or marketing will not be accepted. A PPA remains in effect until terminated by either the manufacturer or the Secretary of HHS. It is not automatically terminated if a manufacturer terminates its Medicaid rebate agreement. or PPA Addendum buttons to view a PDF version of either document.